Chronic Spontaneous Urticaria: Entering a New Era of Innovation and Therapeutic Possibilities | Competitive Intelligence
CSU treatment evolves beyond anti-IgE biologics as novel drugs, biosimilars, and oral therapies reshape the competitive landscape
AUSTIN, TX, UNITED STATES, May 28, 2025 /EINPresswire.com/ -- The chronic spontaneous urticaria (CSU) space is witnessing a pivotal transformation. Long recognized as a frustrating and poorly understood skin condition, CSU is now at the center of a therapeutic renaissance, fueled by cutting-edge biologics, advanced small molecules, and a sharpened industry focus on targeting non-responders and refractory cases.
CSU is characterized by the sudden, recurrent appearance of hives, angioedema, or both, persisting for six weeks or more-often without any identifiable external trigger. Affecting nearly 1% of the global population, it tends to strike individuals in their prime years, most commonly between the ages of 20 and 40. While some experience symptoms for just a few years, others endure fluctuating episodes over a decade or more. Women are disproportionately affected, with incidence rates nearly double that of men.
Book You CI Consultation Call for Free: https://www.datamintelligence.com/strategic-insights/ci/chronic-spontaneous-urticaria-csu
This previously underserved market is now drawing heightened interest from global pharmaceutical leaders, driven by significant unmet needs and a growing patient base. Traditional antihistamines and the widely used anti-IgE biologic, omalizumab (Xolair), have been foundational treatments-but their limitations are increasingly apparent. A substantial subset of patients remain symptomatic despite these therapies, highlighting the urgent demand for more personalized, effective, and convenient options.
A Therapeutic Landscape in Motion
Approved therapies for CSU have evolved beyond antihistamines and Xolair, which remains the gold standard due to its robust real-world data and clinician trust. However, the competitive pressure is intensifying. A new wave of therapies is being designed to fill critical gaps, offering hope to patients who have historically had few alternatives.
Sanofi and Regeneron’s Dupixent (dupilumab), already a blockbuster in other atopic diseases, is now being positioned for biologic-refractory CSU. With its mechanism targeting IL-4 and IL-13, it has demonstrated promise in addressing type 2 inflammation that plays a role in certain CSU patients.
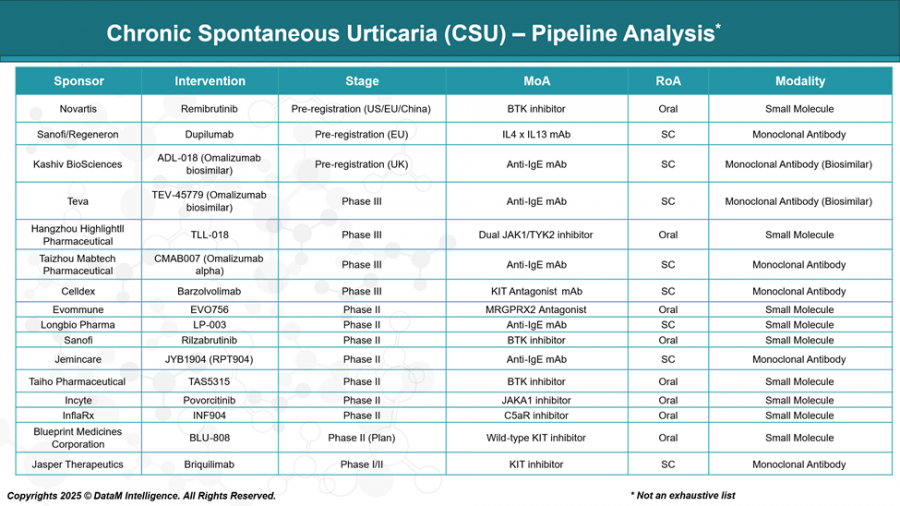
Meanwhile, Novartis is preparing to introduce Remibrutinib, a BTK inhibitor that represents a new class of oral small molecules in CSU. Designed specifically for antihistamine-refractory cases, this candidate could be a game-changer for patients seeking alternatives to injectables.
And it doesn't stop there. Biotech innovators are developing therapies that target other key pathways in CSU pathogenesis, such as mast cell activation, JAK1/TYK2 inhibition, and the KIT receptor. Candidates like Barzolvolimab (Celldex), TLL-018 (Hangzhou Highlightll), and CMAB007 (Taizhou Mabtech) bring novel mechanisms of action that aim to redefine how CSU is managed across varying patient profiles.
Biosimilars: Disruption with Access and Affordability
The rise of biosimilars in the anti-IgE space is reshaping market dynamics. Players like Celltrion (OMLYCLO), Kashiv BioSciences (ADL-018), and Teva (TEV-45779) are introducing cost-effective versions of omalizumab, promising broader patient access without compromising efficacy. These products, many in advanced stages of regulatory approval, are likely to erode market share from branded biologics and increase pricing pressure across the board.
This biosimilar wave is not merely a cost play. With proven interchangeability, favorable safety profiles, and growing physician confidence, they are expected to become integral components of treatment protocols, especially in cost-sensitive markets.
Strategic Differentiation: The New Standard for Emerging Therapies
Emerging CSU therapies must meet a higher bar than ever before. Speed of action, durability of response, convenience, and safety have become non-negotiable for success. According to analysts tracking the market, the most competitive agents will be those that can:
• Deliver rapid relief (within 1–2 weeks)
• Sustain remission over six months or more
• Offer convenient oral or monthly dosing
• Demonstrate efficacy in biologic non-responders
• Maintain a clean safety profile with minimal immunosuppression
• Target novel mechanisms beyond the conventional IgE and IL-4/13 pathways
• Additionally, the rise of companion diagnostics and biomarker-driven personalization is expected to further optimize outcomes. By identifying likely responders in advance, developers can strengthen their value proposition to both clinicians and payers, accelerating adoption.
Ask for Free Sample of Chronic Spontaneous Urticaria (CSU) Competitive Intelligence : https://www.datamintelligence.com/strategic-insights/sample/chronic-spontaneous-urticaria-csu
The Competitive Pulse
While Xolair continues to dominate with over a decade of physician loyalty and strong efficacy data, it now faces formidable competition. Dupixent and Remibrutinib are positioning themselves as high-efficacy alternatives, especially for patients not well controlled with current options. Meanwhile, first-in-class agents like Barzolvolimab are targeting mast cell pathways with the potential to establish entirely new treatment paradigms.
Companies across North America, Europe, and Asia are racing to secure regulatory approvals, with several candidates in pre-registration phases in major markets like the U.S., EU, China, and the UK. This global expansion is expected to significantly widen the therapeutic footprint of CSU treatments over the next three years.
Looking Ahead: Innovation Meets Opportunity
The CSU market is entering a golden age of therapeutic innovation. As more targeted and differentiated agents reach commercialization, the outlook for patients is becoming increasingly hopeful. What was once a chronic, often invisible struggle is now drawing the attention of top-tier drug developers, regulatory bodies, and clinicians alike.
The convergence of scientific discovery, commercial opportunity, and patient need is setting the stage for a transformative era in CSU management. Whether through best-in-class biologics, next-generation small molecules, or cost-effective biosimilars, the market is well-positioned for sustained growth and disruption.
Final Word
Chronic Spontaneous Urticaria is no longer an orphaned disease in the shadows. As the market evolves beyond anti-IgE monoclonals, new entrants with novel mechanisms and compelling value propositions are unlocking the next growth frontier. For stakeholders across the pharmaceutical value chain-developers, investors, clinicians, and patients-the CSU space has never been more dynamic or full of promise.
About DataM Intelligence:
DataM Intelligence 4Market Research LLP is a leading provider of Competitive Intelligence (CI) services, empowering life sciences and healthcare companies with real-time, actionable insights. We specialize in tracking competitor strategies, pipeline developments, clinical trials, regulatory updates (FDA/EMA), and market trends across key therapy areas and indications.
Our offerings include CI insights, commercial analytics, opportunity analysis, KOL overviews, pricing and reimbursement strategies, social media listening, brand/LCM strategies, and market entry assessments. We also offer in-house tools, clinical trial databases, newsletters, and conference coverage to support your strategic decision-making.
With deep industry expertise and a commitment to timely intelligence, we help you stay ahead in a fast-evolving global market.
Read our Related CI Reports:
1. Frontotemporal Dementia | Competitive Intelligence
2. Obstructive Sleep Apnea | Competitive Intelligence
Sai Kiran
DataM Intelligence 4market Research LLP
+1 877-441-4866
email us here
Visit us on social media:
LinkedIn
X
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release